Tampons in daily life
Millions of products are used safely each day and manufacturers ensure that all products comply with all relevant regulations and are safe for their intended and foreseeable uses. Tampons and their raw materials are produced from natural and/or synthetic polymers. Raw materials are selected according to strict quality criteria and during manufacture, rigorous quality control systems and good manufacturing practices are in place to ensure the highest hygienic standards are met.
Before being released onto the market, the composition and safety of all raw materials are reviewed taking into account potential toxicity to the consumer and the environment. EDANA members carry out post-marketing surveillance of their products in which they actively respond to consumer comments to ensure continuously the quality and safety of their products.
To further strengthen the industry’s safety efforts and enhance consumer confidence. EDANA and its members have embraced a new level of action and transparency on levels of trace substances with the voluntary EDANA Stewardship Program AHP.
List of important documents:
- Tampon infographic
- Tampon component table
- EU Tampons Code of Practice
- Absorbent Hygiene Products (AHP) Labelling Guidelines
- EDANA Guidelines for Testing Menstrual Products guidance document
- EDANA’s Safety and Regulatory Supply Chain Information for Absorbent Hygiene Care Products
- Exposure-Based Risk Assessment (EBRA) menstrual products
- The voluntary Stewardship Program CODEX

Composition of tampons
Some misconceptions revolve around how tampons are manufactured and what their components are. The reality is that tampons are complex, multilayered products made by assembling various components, each of them with various functions.
The Tampon infographic and Tampon component table present the different components used for the manufacturing of a tampon as well as the different parts composing a tampon, you can easily download a copy.
The guidance document on AHP labelling gives details on transparency and clarity.
Safety and labelling of tampons
Tampons are made of well-proven materials that are also used in a variety of other everyday products which have proven safety profiles. The raw materials are carefully selected for the highest quality and undergo extensive safety evaluation to ensure a lack of harmful effects and good tolerability before they are approved and used during manufacturing. In order to deliver a safe product, tampons are made under high quality production control standards including a series of checks and tests based on company quality assurance systems and user monitoring programmes.
Within the European Union tampons must comply with the General Product Safety Directive that holds manufacturers responsible for providing consumers with products that are safe to use. You can read more about the legal requirements and the voluntary guidelines applied in the industry in EDANA’s Safety and Regulatory Supply Chain Information for Absorbent Hygiene Care Products.


In addition, tampon manufacturers in Europe follow the “ EU Tampons Code of Practice”, or a national equivalent.
The Code of Practice was published to harmonise relevant consumer information in all EU countries, irrespective of the tampon brand used. Key elements of the Code of Practice include :
- information and advice on correct tampon use,
- information about Toxic Shock Syndrome,
- a standardised test method to ensure the absorbency ratings are consistent across all manufacturers/brands. This Syngina test method, NWSP 350.1.R1 (20) is available upon request.
- a droplet system that categorises the absorbency of tampons into six classes.
To ensure safe use of the product, manufacturers recommend reading the detailed instructions inside the packaging, as well as information on the packaging itself.
Menstrual products testing guidelines
The guidance document aims to meet the demand for relevant, accurate, reliable and comparable tests, for performance standards as well as substance testing across a range of menstrual products.
The guidelines are intended as a best practice tool for third-parties to consider to ensure the scientifically sound testing of menstrual products and meaningful results. They were developed by a group of experts from manufacturers of feminine hygiene products, their suppliers, and leading test institutes with expertise in testing femcare products. The guidelines are comprehensive, covering all types of femcare products (panty liners, tampons and sanitary pads), all forms of testing and each step involved in a test, from design and methodology to the interpretation and communication of results. Statistical methodology and technical parameters impacting test results were taken into consideration for the development of the guidelines.
In addition to technical and methodological guidance, the guidelines remind organisations conducting tests of several key principles. For instance, third party tests intended to serve as the basis for consumer information must include a user trial. Also, to ensure that the products are tested in an accurate manner and that the results reflect the actual experience of consumers, it is recommended to consult manufacturers and to conduct tests in a laboratory experienced in testing femcare products. Practical information such as contact details for the main manufacturers that are members of EDANA are included in the annexes, along with suggested laboratories that are EDANA members, standard method references, and questions for user trials. Any feedback and input to the guidelines is welcome and will be taken into consideration in future versions.
Tampons and trace levels of impurities

The EDANA Stewardship Programme for Absorbent Hygiene Products (AHPs) is a voluntary initiative launched by the nonwovens industry in July 2020. It builds on a series of pre-existing voluntary initiatives under EDANA’s Vision for the nonwovens industry to provide transparency and reassurance for consumers regarding trace levels of impurities found in these products. Absorbent Hygiene Products include baby diapers, menstrual care products, and adult incontinence products.
Signatories to the programme undertake:
- to monitor the presence of a defined list of trace chemicals in AHPs;
- to take action to ensure that they do not exceed agreed guidance values and that these guidance values are lower than those required by existing EU and national legislation;
- to reinforce transparency by publishing product composition to enable consumers to make informed choices;
- to take part in communication activities across the EU to further enhance consumer understanding of trace level impurities.
The Stewardship Program allows companies to voluntarily go beyond current EU and national legislation and the participants are free to go beyond the established criteria of the Stewardship Program based on their specific quality assurance processes.
The core of the EDANA Stewardship Programme is the voluntary CODEX, which features:
- an evolving industry-wide list of chemicals (including, but not limited to, PAHs, PCBs, dioxins, furans, phthalates and formaldehyde). These are not intentionally used to manufacture absorbent hygiene products (AHPs) but may be present in trace amounts
- guidance values based on existing related regulations, regulatory guidance, related existing standards or industry experience. These guidance values should not be exceeded
- consumer relevant test methods for assessing the presence of the trace chemicals
- Exposure-based risk assessment (EBRA) is currently the proposed method of choice, as a scientifically sound approach using realistic exposure parameters. The guidance values do not affect the requirement to have the necessary safety assessments for individual products to comply with the General Product Safety Regulation 2023/988 (EU). The open development of guidance values follows a tiered approach and evolves over time as new scientific data and insights are available or regulatory limits are updated. A Scientific Review Panel provides expert advice on the EDANA Stewardship Programme CODEX. This Scientific Review Panel has also developed a compendium with key facts around AHPs, from supply to manufacture, safety evaluation, market surveillance, with focus on the CODEX guidance values and the analytical extraction methods.
General information about tampons and how to use them safely
Find the text below in your own language by clicking on the button corresponding to your language
General information
Tampons are products used to absorb menstrual blood during your period and are inserted inside the vagina. Their absorbent material is cotton, viscose (rayon) or a blend of the two. Tampons are designed to be inserted either by using a plastic or cardboard applicator or without an applicator (by using only a finger). All tampons are provided with a string to ensure a safe removal. Some also have an additional outer cover layer to help with insertion and removal.
Every menstrual flow is different, changes from day to day throughout each period and can differ during your menstrual lifetime. Therefore, tampons are made in a range of absorbencies/sizes to help manage these different flows. Always choose the lowest absorbency to suit your menstrual flow needs.
Every pack of tampons has a chart describing the tampon absorbency in droplets and in grams and linking them to a recommended menstrual flow.
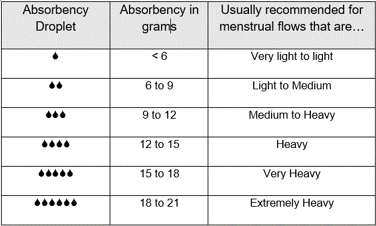
Attention: these are general recommendations valid for all kind of tampons. For specific instructions, including details on insertion and removal, how often to change and how long you should wear a tampon for, please refer to the packaging or product information of the tampon brand you are using.
- Wash your hands before and after inserting a tampon.
- Use the lowest absorbency/size tampon to suit your flow.
- Use only one tampon at a time.
- Remember to remove the last tampon at the end of your period.
Read about Toxic Shock Syndrome


